We use cookies to make your experience better. By continuing to browse or use our website you are agreeing to our use of cookies. Learn more
Safe Disposal of LiPo Batteries
By John Julian (AKA JJ604 on RCGroups)
Introduction
Lithium Polymer (LiPo) batteries have largely replaced not only other types of battery but also internal combustion engines as the preferred energy source for small and medium size model aircraft. We also see increasing numbers used in large electric aircraft.
That leaves us with an ongoing problem: how to get rid of old LiPo batteries in a safe and environmentally responsible manner.
The Salt Water Method and its Hazards
It is commonly accepted that the preferred way to dispose of a model LiPo pack is to immerse it in a container of salt water for an extended period (from a day or two to a couple of weeks). After this it is assumed that the voltage across the connector is essentially zero and that the battery can safely be disposed of in the trash, as it is now supposedly inert. Indeed, many clubs have just such a salt bath at the flight line or clubhouse for exactly this purpose.
This document explains why salt water disposal on its own is, in most cases, a bad idea, and why even dead LiPos are not as hazard-free as sometimes suggested.
On the surface, the salt water bath idea seems reasonable. After all, salt water conducts electricity, so dropping the battery in salt water should discharge it. But this ignores the process called electrolysis. Salt water is indeed conductive and electricity will pass through it between the battery terminals, discharging the pack. BUT the combination of electric current and dissolved salt will also attack many metals, including the tabs that actually conduct the electricity between the LiPo cells. Once those tabs are destroyed; the circuit is open and there is no more discharge. It’s a race between the discharge of the pack and the destruction of the metal tabs, and in many real-world cases the tabs are gone before the cells are completely discharged. Then you are left with a charged pack and no way to discharge it. Worse, the pack appears completely discharged, because when you measure the voltage on the power leads it reads zero.
If a salt water bath is a bad idea, why is it so commonly advocated and where did the recommendation originate?
In the very early days of LiPo adoption, the extremely knowledgeable Fred Marks (founder of FMA Direct) was a distributor of Kokam batteries, the first readily available LiPo packs for model use. He wrote a user manual in which he recommended ultimately disposing of the batteries in salt water. In addition, the document also called for the envelope of each cell to be punctured so the salt water would get inside the pack and “neutralize” the electrolyte. But these measures were to be taken only AFTER the battery was fully discharged.
Here is the exact wording of that section from the 2003 version of the Kokam guide.
12. Dispose of cells/packs as follows:
a. Discharge: with the cell/pack in a safe area, connect a moderate resistance across
the terminals until the cell/pack is discharged. CAUTION: cell/pack may be hot!
b. Discard:
- NiMH: place in regular trash.
- NiCd: recycle (cadmium is toxic).
- LiPo: puncture plastic envelope, immerse in salt water for several hours, place in regular trash.
Some major vendors at the time gave essentially the same recommendations as Kokam, with a few variations. For example, Thunder Power recommended leaving the battery in the salt bath for 2 weeks, but only after total discharge using a resistive load such as a car light bulb for 24 hours.
To emphasize, there was one absolutely critical point in this procedure: the battery was to be completely discharged before opening it up.
Puncturing of the cells in this way to allow the salt water to neutralize the electrolyte was at that time considered an effective way to dispose of a LiPo battery. Even then it was recognized that puncturing the cells of a charged LiPo battery can be extremely dangerous; a damaged LiPo with appreciable charge can explode or burst into flame violently. Hence the instructions to discharge fully first.
It was soon recognized that these instructions were open to serious misunderstanding. Because of the potential danger, after 2005 Kokam no longer recommended using the salt water bath with cell puncturing, even with a prior discharge.
However, the damage had been done. People did not read the original recommendations carefully and the “salt water bath” method as a complete disposal technique, without the necessary precursor discharge, became an Internet “Truth”. Indeed, it was even reproduced by some LiPo vendors in their manuals.
What exactly does salt water do to a LiPo?
The “salt water” method does nothing to properly neutralise a LiPo. It may actually make things worse.
Salt water is Sodium Chloride (NaCl) dissolved in water (H2O). The NaCl ionises, causing the solution to become electrically conductive, allowing the battery to drain. If the cells are punctured, the salt water can interact with the electrolyte within. NaCl is chemically inert in this context and does nothing to “neutralise” the LiPo contents. The water, however, can react with some chemistry in the cells.
With the electrolyte in direct contact with water, a number of chemical reactions can occur, and for at least three of the possible reactions between the electrolyte and water there is potential to produce Hydrogen Fluoride, which in aqueous (water-based) solution becomes Hydrofluoric Acid. This is highly corrosive and toxic and can have very nasty effects on the human body. In this case the concentration is very low, but still not something to mess with.
In addition to the HF issue, some components of the electrolyte within LiPo packs are directly toxic to the human body or considered a health hazard in themselves. Ethers like 1,2-Dimethoxyethane (DME) are one example. In LiPos, the amounts are very small, but not totally harmless. Normally, these substances are safely contained in the fully sealed “pouch” package, but if you rupture that seal they can escape.
So, using a salt water bath for disposal is a bad idea in most cases.
As discussed later, however, a salt bath may be the only option for rendering the battery relatively safe if the battery is so damaged by impact or other events that no other means of discharge would work. However, its use in such cases needs great care and consideration for safety.
How to Deal with Defunct LiPos
Intact Batteries
Here we are considering batteries that have not suffered serious physical damage (for example, from a crash). They may be significantly puffed (see Appendix B) and/or have cell voltages that are drastically out of balance, or they may just be too far down on performance to continue in active use. The point is that the cells are still sealed, and both the main battery leads and the balance leads are functional, thus allowing the battery to be safely discharged.
The basic recommendation is that a battery for disposal be discharged as far as possible – certainly to below 2V/cell and preferably to 1V/cell or even below. To minimize any risk, this discharge should be done at a rate well below 1C and ideally not exceeding about 0.5C.1 While some swelling of the pack is likely, such a relatively low current makes serious puffing or heating highly unlikely. The discharge process may take two hours or more.
Discharge rates above 1C progressively increase the risk of major swelling, cell rupture or even fire.
(Note that some people recommend an even more cautious approach to discharge. A rate of 0.1C to 0.2C with discharge terminated at about 2V/cell should avoid almost any chance of the cell puffing or heating seriously.)
Regardless of rate, any major discharge should be done outdoors, in a location where there is no risk of a burning pack starting a fire.
1The C-rate is the actual discharge current in Amps divided by the pack C-rating in Amp-hrs. So, a 2200mAh (2.2Ah) pack discharged at 1.1 Amps is being discharged at 0.5C rate.
Possible methods of discharge
For many LiPo packs, the NiMH/NiCd discharge function of a multi-chemistry charger works fine. The NiMH/NiCd cycle normally allows discharge to about 1V/cell which is in line with the outcome discussed above.
Connect the LiPo to the charger as usual but without connecting the balance leads. Set the charger to discharge mode for a NiMH or NiCd battery. Select a current that is equivalent to 0.5C or less. For example, for a 2200 mAh pack, set a discharge current of up to about 1.1 Amps.
Most chargers will also require you to set a cut-off voltage for the discharge. Some express this as the voltage per cell, while others ask for the total battery voltage.
Some chargers simply will not permit the NiMH/NiCd discharge function to be used in this manner, in which case, other means of discharge have to be used.
For most discharge requirements, it’s hard to beat the humble incandescent 12-volt automobile bulb. These are available with various power ratings. It is common, for example, to use the typical 21 Watt, 12V car tail light bulb. This will provide about a 1.7 Amp load on a 3S LiPo pack, a discharge rate that is a bit higher than recommended but still reasonable for a 2200 mAh battery. And this is one load that even tells you when the process is done – the light goes out. ????
By assembling a number of suitable bulbs in series and/or parallel you can make up a load that will completely discharge almost any size battery at the recommended rate of about 0.5C or less. Details are provided in Appendix A.
To discharge your battery, just plug it into the load, put it somewhere safe and leave it until the bulb goes out. “Somewhere safe” means a place where, if by any chance the pack overheated or caught fire, no serious damage would occur. Outdoors in a cinder block, in a barbeque, or on a gravel path are all popular discharge locations.
After the light goes out, leave things for another half hour. Then check the voltage to make sure the battery is completely discharged (2V/cell or lower). Now, cut off the battery connector (one wire at a time), strip the wires and twist them together (this may produce slight sparking). Then dispose of the now completely safe battery.
There are also a number of purpose-designed LiPo dischargers. However, most of these are primarily designed for reducing the LiPo to storage voltage (3.8 or 3.85V/cell). Many of them cannot be set to a low enough cut off voltage to achieve full discharge (2V/cell or lower) and therefore are not useful for disposal of batteries.
NOTE: It is likely that the pack will puff (swell) somewhat or warm up toward the end of the discharge. This is not normally a cause for alarm and cannot be avoided. For more information on LiPo puffing see Appendix B.
Dealing with damaged batteries
Here we are referring primarily to batteries that have suffered serious crash damage or have been subject to electrical abuse, such as over-discharge to the point of cell rupture. The key problems are that the connections normally used for discharge may not be available and possibly that some cells are burst or split.
This is a tricky one, as you cannot do a simple discharge if a normal voltage (e.g., 9 to 12.6 volts for a 3S pack) does not appear across the discharge connector. This means that an internal connection is broken and the battery cannot be discharged normally.
There are two likely scenarios:
No voltage on main connectors, but balance connections intact
If the cells appear to be intact, verify that the balance leads are still connected by measuring the voltage between every adjacent pair of pins. If this is successful, you can discharge the battery using the two end pins on the balance connector. Alternatively, you can discharge each cell individually using a suitable resistive load across each pair of adjacent balance pins. Do not exceed about 0.5C discharge, to a maximum of 4 Amps (balance connectors are OK up to 4 Amps load in this particular one-use-and-discard case). Suitable values for the resistor can be calculated from the information in Appendix A.
- If there is significant visible physical damage to one or more cells and/or some balance connector pins show zero volts, do not discharge either individual cells or the battery as a whole.
What to do with a pack that cannot be discharged safely?
Place in a strong sealed plastic bag and deliver to a hazardous waste facility. Do not place in regular garbage.
Before such disposal, you may wish to minimize any potential hazard by immersing the battery in a salt water bath for at least two weeks. The salt bath will at least partially discharge the individual cells and may even do so completely, even if the connections between them are broken or eaten away. Note that you will not be able to tell - as you cannot measure the pack voltage.
Disposal of LiPo packs
Once the battery for disposal has been fully discharged as discussed above (i.e., to less than 2V/cell), it can be considered essentially inert. Since LiPo batteries contain no significant quantities of mercury, lead, cadmium or other highly toxic substances which will leach into landfill over time, they do not represent a risk of air or water contamination and may, if the applicable regulations permit, be treated as regular trash.
In many jurisdictions, however, batteries of all types are considered, by definition, to be hazardous waste and must be dealt with accordingly (usually meaning they must be taken to some type of public or private pick up point or disposal facility).
Consequently, for batteries that are fully discharged (to 2 v/cell or less):
- In places where regulations do not classify discarded lithium batteries as hazardous waste, disposal may be as simple as dropping the battery in the regular (nonrecyclable) garbage.
- If the jurisdiction prohibits disposal of lithium batteries in this way, they must be treated as hazardous waste and dealt with as specified in applicable regulations
Any battery that cannot be discharged represents a risk, not due to toxicity but because of the electrical energy it contains. Such damaged batteries should be placed in a plastic bag to prevent short circuits and delivered to the local hazardous waste system.
Can our LiPos be recycled?
Clearly, the most desirable end of life treatment for used batteries would be for the valuable materials, including lithium, to be recycled into new batteries. Unfortunately, at present, recycling technologies are limited in efficiency and facilities are not widely available, being found only in parts of Europe, North America and Asia. One study that looks at the problem is called “The environmental impacts of recycling portable lithium-ion batteries”.2
2 Anna Boyden, The environmental impacts of recycling portable lithium-ion batteries, A Thesis submitted in part fulfilment of the degree of: Bachelor of Engineering, Department of Engineering, Australian National University, December 2014
Check with local waste disposal authorities regarding the availability of relevant recycling programs.
Just a final word. Although the disposal of model LiPo packs is background noise in relation to the overall issue of dealing with used batteries from electric traction and mobile devices, as a matter of principle modellers should support efforts to minimize the environmental impact of lithium battery disposal.
One important way to do this is to treat batteries in a way that maximizes their useful life and thus minimizes the need for replacement. Essentially, this boils down to avoiding overcharging, over-discharging and exceeding the battery’s ability to deliver current (its real “C” rating).
Bottom Line
- Never, ever puncture or slash the cells open. It is a really bad idea. The stuff inside is potentially hazardous and it could lead to fire, smoke and poisoning.
- For safe disposal, discharge a discarded LiPo to 2 V/cell or below at a recommended rate of no more than 0.5C.
- Dispose of the battery at a recycling station or appropriate waste disposal centre.
- If cells are damaged, use nitrile or neoprene gloves and safety glasses. Note that latex gloves are not satisfactory, due to the organic solvents in the electrolyte.
- Avoid contact with cell contents, electrolyte mist, or smoke from a burning cell. If necessary, seek medical assistance.
- Never inhale smoke from a burning cell. If you do, seek medical assistance immediately.
- Any battery that can’t be adequately discharged should be wrapped in plastic before delivering to a hazardous waste centre.
Appendix A: Discharge through a Resistive Load
Solution 1: Automotive Light Bulb
There are a number of automotive incandescent light bulbs (not LEDs) which can be used as a battery discharge load.
If you have access to an ammeter or wattmeter, you can choose a bulb with suitable specifications to drain the LiPo pack at the recommended rates. Even if you don’t have such a meter, you can use the very common dual-filament combined stop and indicator light incandescent bulb for this purpose, as explained below.
Automotive bulbs are normally designed to run on a nominal 12 Volts although the actual voltage in a car may vary up to 14 V or more; the bulbs are designed to tolerate that level without failure. The traditional indicator/stop light bulb has a resistive filament which glows white hot. It has been around for many years and is ideal for this task. It is cheap, robust and happily operates at reduced voltage, which some other bulbs do not. The most common type is rated at 12 V, has a bayonet fitting and two filaments of 21 Watts and 5 Watts for stop/turn signal and running light respectively. A variety of related bulbs are available rated at 12-14V with filaments of 18-27 Watts and 5-7 Watts. Any of these can be used, depending on the pack capacity, as the discharge application is not critical.
Other styles of bulb such as headlights and halogen bulbs and the wedge style of stop/indicator bulb are generally less suitable and/or significantly more expensive. The basic incandescent dual filament auto bulb rarely costs more than two or three dollars.
Obviously, you can’t connect a single 12V bulb to a LiPo pack of more than three cells (3S). A 4S pack has a maximum voltage of 16.8V and the bulb will soon blow.
Consequently, to discharge packs above 3S, you need to put two or more 12 V bulbs in series. In fact, two 12V bulbs in series are a practical discharge load for most of the LiPos we use.
Specifically, you can combine two of these bulbs with two LiPo connectors into a very useful “universal” discharger for 1-6S packs, as shown below.
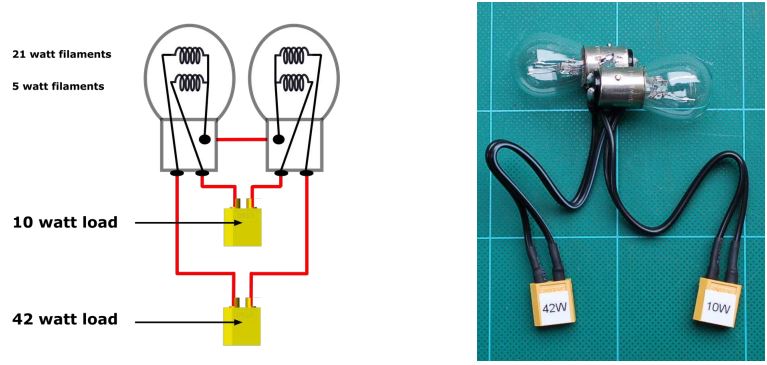
In practice this is a cheap and simple configuration that safely covers discharge requirements for the most common packs.
The table shows actual measured resistance values. Note that a light bulb does not follow Ohms Law, as the effective resistance is highly temperature dependent. Because we are not using these bulbs at their rated voltage, they run cooler than expected.
In practical terms, the 2x 5Watt connection serves for 1-6S packs under about 2200mAh and the 2x 21Watt connection handles bigger ones. If in doubt use the smaller (2x 5 Watt) load and leave it to discharge for as long as it takes.
If you use packs over 6S, then use three such bulbs in series (good up to 9S) or four (good up to 12S) and plug into the appropriate connector for the pack capacity, just as for lower cell counts.
If you use very large capacity packs and don’t want to wait a long time for the discharge to complete, you can put two bulb assemblies in parallel to double the current.
If you use very small packs you can consider using lower wattage bulbs, but a wire wound power resistor might be more convenient, since the power being dissipated is reasonably small (see Solution 2, below).
Solution 2: Resistor
A wire-wound resistor can be a convenient means of discharge, especially for small LiPos.
To calculate the size for a 0.5C discharge rate:
- Discharge current: Battery Capacity in Ah (Amp-hours) divided by 2.
- Resistance required: 3.7V * Number of cells divided by Discharge Current
- Minimum power rating of resistor: Watts = Resistance * Amps * Amps
Example1
For a 3S 1000 mAh (1 Ah) pack:
Discharge current = 0.5A
Resistance = 3.7*3/0.5 = 22 Ohms
Minimum power rating of resistor: 22*0.5*0.5 = 5 Watts approximately
Example 2
To discharge one cell of a damaged 2200mAh pack through the balance connector pins:
Discharge current = 1.1 A
Resistance = 3.7*1/1.1 =3.3 Ohms.
Minimum power rating of resistor: 3.3*1.1*1.1 = 4 Watts approximately
Appendix B: Cell Puffing and its Causes
Puffing, or battery swelling, is the main visible sign of degradation in LiPo batteries. There are two types of puffing that can occur during use, storage or disposal of batteries: reversible and permanent.
Reversible puffing (goes away after cooling).
This can occur if you overstress a pack during discharge by using too high a current. It happens because the high current discharge heats the battery beyond the point where some of the liquid electrolyte mixture turns into a gas phase. After cooling the puffing subsides again as the gas condenses back to liquid. There is a high probability however that the pack has been significantly degraded by this experience; such degradation is cumulative.
Permanent puffing
There are two main causes of permanent puffing:
- Overcharging, storing at full charge, or overheating (which is often due to exceeding the battery’s maximum safe discharge rate). In broad terms, the electrolyte and a layer on the anode decomposes, producing mostly CO2, and some usable lithium is lost. The resulting puffing and loss of performance is cumulative over subsequent incidents and is a common cause of LiPo degradation.
Over-discharging. When a LiPo cell is discharged below about 3.0V/cell under load, the cathode material may decompose and produce (among other things) oxygen. This then reacts with other cell contents - sometimes violently. This is a common cause of cell failure that can result when users rely on Low Voltage Cut-off in the ESC to signal time to land.
During final discharge prior to battery disposal, some LiPo puffing frequently occurs as the voltage drops. If moderate, it is no cause for concern, but extreme puffing can be the prelude to thermal runaway and fire. This is why high rates of discharge should be avoided when preparing for disposal.

 Europe
Europe